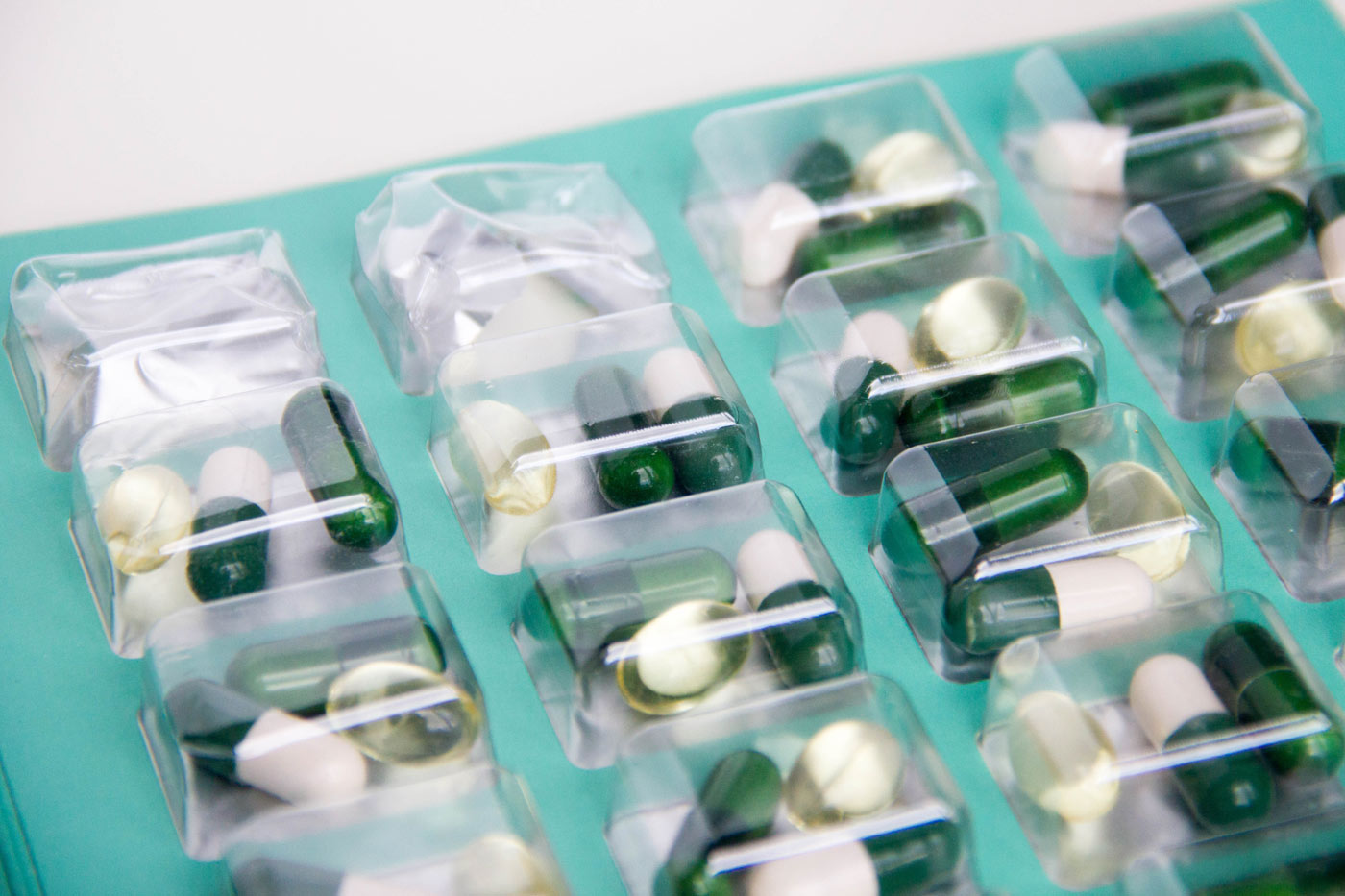
Novartis Pharmaceutical and its generics division, Sandoz, have voluntarily recalled about 470,000 blister packs of haloperidol and other medications with similar blister packaging. Haloperidol is an antipsychotic medication that is used to treat schizoaffective disorders, Tourette’s disorder and children who are hyperactive. It was a child who triggered the Novartis and Sandoz drug recall when a blister pack containing haloperidol tablet of the medication was opened and ingested. A blister pack is a type of packaging in which a product is sealed in plastic, often with a cardboard backing. Novartis advised that it had voluntarily notified the Consumer Product Safety Commission (CPSC). Federal law prohibits the sale of products that have been voluntarily recalled by a manufacturer after a public recall.
Unsafe Packaging
The Novartis drug recall was announced by the CPSC and the Food and Drug Administration as soon as they learned that a child was able to open a blister pack and remove and swallow the medication. The CPSC explained that “The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act, posing a poisoning risk if swallowed by children.” The CPSC was silent on whether the issue of how or whether the child had been affected by the drug. As per the Sandoz drug recall, the company holds itself out as a “seal of quality.” Novartis remarked that both Sandoz and Novartis, “take our responsibility for consumer safety very seriously.” The quality of the haloperidol was never at issue. Patients were encouraged to continue to take their medications as prescribed. It was the safety of the packaging of the medication that came to issue. Along with haloperidol, other medications involved in the Novartis drug recall that were recalled as a result of blister pack issues included the following:
- Risperidone ODT
- Donepezil ODT
- Naratriptan
- Ondansetron tablets
- Zofran ODT
- Azithromycin
- Imipramine
- ISDN
- Perphenazine
- Entresto
The Doctor Reddy’s Settlement
Federal regulators take a dim view on poorly packaged drugs. In December of 2017, a total of $5 million was paid to the U.S. Department of Justice by Dr. Reddy’s Laboratories in order to settle a protracted investigation into claims that it knowingly distributed prescription medications in the United States that weren’t tested for safety around children. The investigation lasted for more than six years. Government attorneys alleged that Dr. Reddy’s engineers were aware of the fact that four of the drugs that it manufactured didn’t meet federal child-resistance laws. After that, Dr. Reddy’s allegedly changed the packaging and sold the product with untested packaging.
Other Recent Packaging Recalls
Merck was involved in two packaging recalls in 2014 and 2015. Both were voluntary. The 2014 recall involved a packaging defect with Liptruzet, a drug used to lower cholesterol levels. That recall involved a possible defect that might have allowed air and moisture to leak into and affect the efficacy of the drug. The 2015 recall involved 276,000 bottles of a cancer drug known as Temodar and generic temozolomide capsules. Although packaged in a bottle, the bottles had caps that could crack and disable child-proofing.
Although child-resistant packaging can reduce the risk of children ingesting dangerous drugs, the CPSC states that “There is no such thing as child-proof packaging. So you shouldn’t think of packaging as your primary line of defense. Rather, you should think of packaging, even child-resistant packaging.” In order to determine whether packages can be opened, testing is performed with actual children. The rules that define what child-resistant packaging is are found in the Code of Federal Regulations, Title 16 Part 1700.
Learn more about Drug Safety News.
