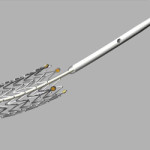
 Imagine purchasing a drug to combat Peripheral Artery Disease so blood flow in the legs can improve, and the exact opposite happening. The FDA, in regards to Cook Medical’s Zilver PDX drug eluting peripheral stent has issued a recall on the popular medical device. The recall has been issued due to a defect in the stent’s delivery system which the company received numerous complaints about.
Imagine purchasing a drug to combat Peripheral Artery Disease so blood flow in the legs can improve, and the exact opposite happening. The FDA, in regards to Cook Medical’s Zilver PDX drug eluting peripheral stent has issued a recall on the popular medical device. The recall has been issued due to a defect in the stent’s delivery system which the company received numerous complaints about.
Why is There a Zilver Stent Recall
Based on Cook Medical’s investigation of the Zilver stent, it was found that during the delivery system, separation at the tip of the catheter occurred. Acatheter is a tube inserted through an opening into a body cavity which removes fluid. This issue occurred from the delivery system, and not from the drug itself, which remains safe. There are potential adverse effects, which could be extremely dangerous.
Adverse Effects of the Zilver Stent
There are numerous adverse events involving the Zilver stent, which could occur with the breakage of the catheter. According to n FDA press release, such events include but are not limited to surgery to remove the catheter tip, vascular blockage from loose tips, or in some cases, cardiac arrest. As of today, one death has already occurred and due to these serious risks, the FDA issued the Zilver stent recall as Class 1.
Finding the Problem in Manufacturing the Stent
In late April, Cook Medical sent an urgent medical device recall to all customers affected by the Zilver stent, which in detail explained the defects of the product and the actions that will be taken. Cook has since identified the internal component triggering the problem and they are modifying its manufacturing process to avoid making the same error. The company says its bare metal version of the Zilver stent uses a different delivery system and is not included in the recall.
The period of the recall retains to all products distributed from December 13, 2012 through April 16th of this year. The firm says customers who purchased the Zilver stent during the period covered by the recall should return the defected product for credit.
